University of Pittsburgh
Wei Du Hematopoiesis Research Lab
Overview
Dr. Du's research is centered on pathophysiology of hematologic diseases such as bone marrow (BM) failure and leukemia. We investigate the mechanism of hematopoietic stem cell (HSC) mobilization and BM niche engraftment as well as the factors implicated in cell proliferation and apoptosis. Our studies have identified functional interactions between certain factors implicated in cell polarity, adhesion/migration, stem cell metabolism and aging; and have led to numerous peer-reviewed scientific papers in high-impact scientific journals, including Blood, JCI, Nat Communications, Leukemia and so on. Our current research interests include: 1) Study the crosstalk between DDR and immune responses in leukemogenesis; 2) Investigate the systemic immune effects of persistent DNA damage during aging; 3) Understand role leukemia-associated macrophage (LAMs) in leukemogenesis; 4) Define a novel paracrine Wnt5a-Prox1 signaling axis in regulating HSC regeneration under conditions of injury and aging; and 5) Explore the role of major Fanconi anemia pathway in hematopoiesis.
In our research projects, we utilize cellular, genetic and molecular techniques to identify and characterize critical pathways that regulate hematopoietic stem cell functions using knockout (KO) mice and xenotransplant models.Hematopoietic stem cell (HSC) and bone marrow (BM) niche interaction
Description
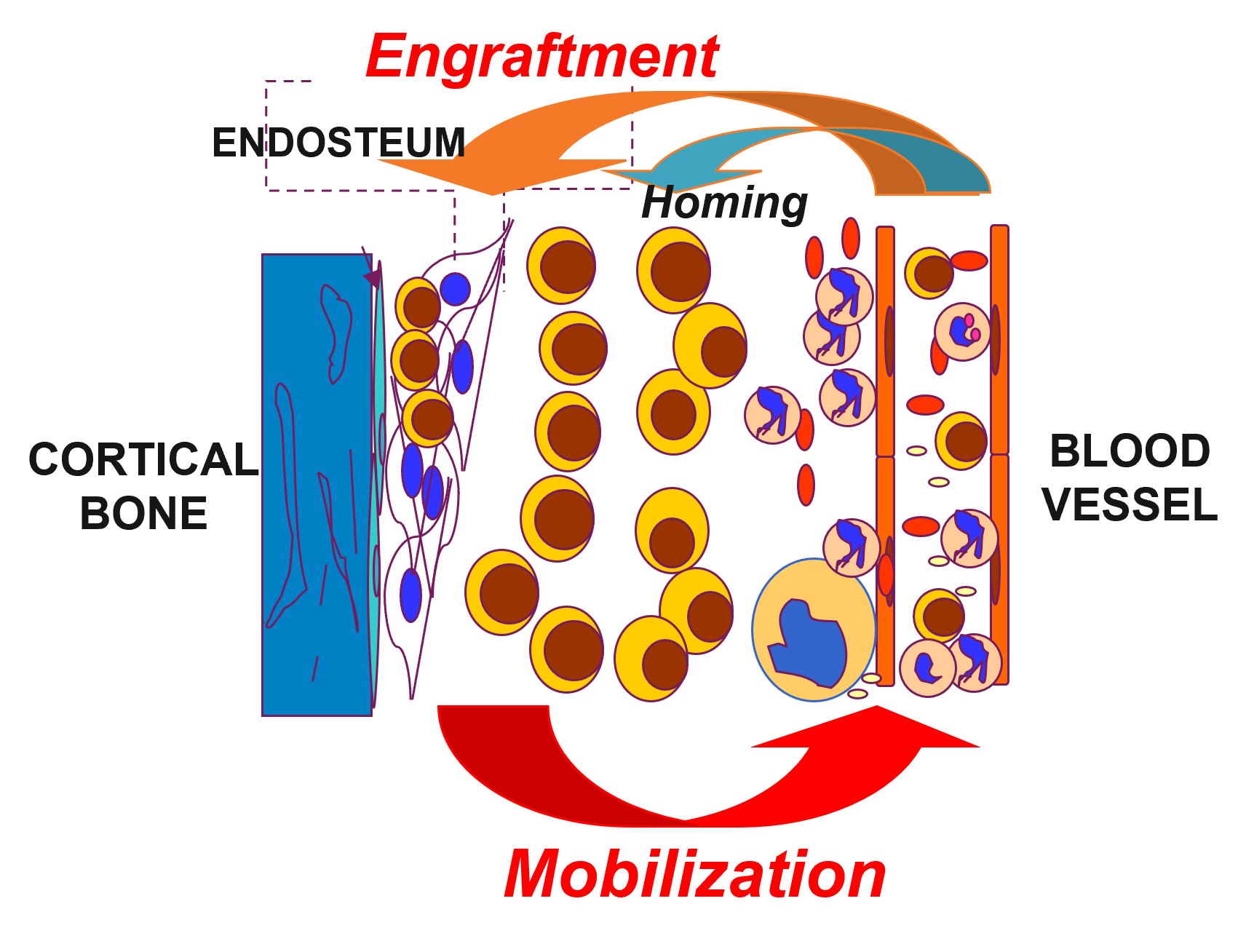
HSCs are a rare population of cells that reside in a unique BM microenvironment (also termed niche), undergo a complex but highly regulated hematopoietic program throughout the prolonged lifespan of mammals. In addition to their multipotency that supports blood cell development, HSCs are characterized by their self-renewal ability, a property that preserves the mostly conserved HSC numbers found in animals and involves asymmetric cell division producing daughter HSCs and progenitors in the BM niche.
Related publications
- Li X, Lin Q, Chowdhury F, Mazumder MH, Du W¥. (2021) Quinacrine-CASIN combination overcomes chemoresistance in human acute lymphoid leukemia. Nat. Commun. 12(1):6936. PMID: 34836965. ¥Corresponding/Senior author.
- Liu W, Du W*, Wang L, Shang X, Ryan MA, Marchioni F, et al. (2019) Rational identification of a Cdc42 inhibitor presents a new regimen for long-term hematopoietic stem cell mobilization. Leukemia.33(3):749-761. * co-first author.
- Du W*, Liu W, Mizukawa B, Shang X, Sipple J, Wunderlich M, et al. (2018) A non-myeloablative conditioning approach for hematopoietic stem cell engraftment in mouse models. Leukemia. 32(9):2041-2046.
HSC regeneration in response to DNA damage and aging
Description
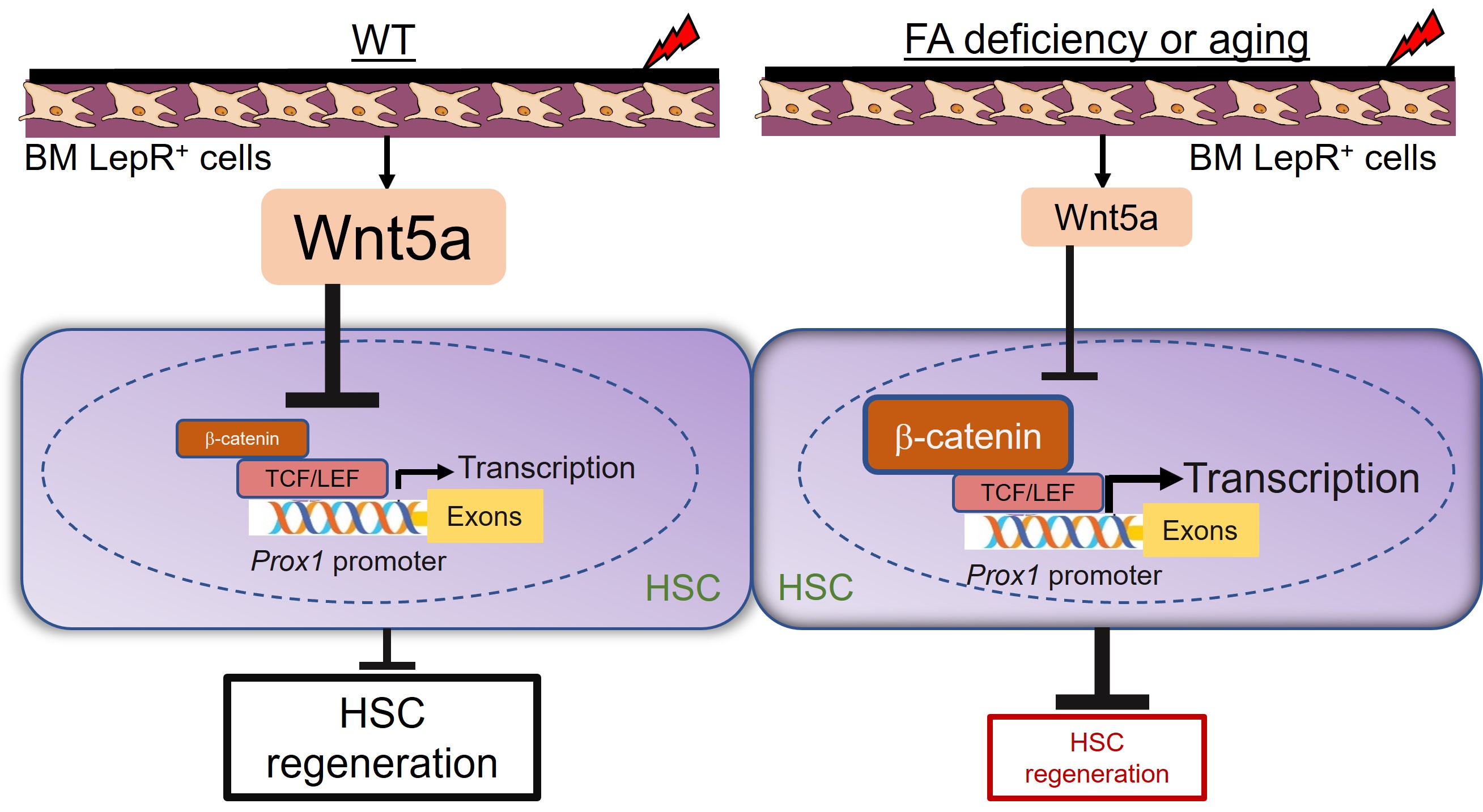
HSC regeneration is the remarkable process by which extremely rare, normally inactive cells of the bone marrow can replace an entire organ under the conditions of injury or harnessed by transplantation.
By using the mouse models of Fanconi anemia (FA) and aging models, we study HSC regeneration under conditions of injury and aging.
Related publications
- Lin Q, Chatla S, Chowdhury F, Atale N, Joseph J, Du W¥. (2022) Hematopoietic stem cell regeneration through paracrine regulation of the Wnt5a-Prox1 signaling axis. J. Clin. Invest. 132(12):e155914. ¥Corresponding/Senior author.
- Du W*, Rani R, Sipple J, Schick J, Myer KC, Mehta P, Davies SM, Pang Q. (2012) The FA pathway counteracts oxidative stress through selective protection of antioxidant defense gene promoters. Blood. 119(18):4142-4151.
Interplay between DNA damage and immune response in leukemogenesis
Description
.png)
DNA damage response (DDR) and immune response are two fundamental processes that play critical roles in the organisms’ genomic integrity and surveillance against tumors. Although DDR and immune response have been characterized extensively, the links between them remain poorly defined. While much attention on the interplay between DDR and immune response has been given to conventional immune cells, a functional connection and coordination between the two processes in hematopoietic stem cells (HSCs) is largely unknown.
The project aims to investigate a previously unknown crosstalk between DNA damage and immune responses in leukemogenesis. Defining the molecular and functional collaboration between diminished DNA damage response resulted from DDR deficiency and dysregulated immune response in the context of leukemia development will open up a new avenue of research designed to target these interacting pathways for developing innovative therapeutic strategies for leukemia treatment.
Related publications
- Li X, Chatla S, Wilson A, Wu L, Atale N, Lin Q, Du W¥. (2022) Persistent DNA damage and oncogenic stress-induced Trem1 promotes leukemia in mice. Haematologica. 107(11):2576-2588.
- Du W*, Amarachintha S, Wilson AF, Pang Q. (2017) The immune receptors Trem1 cooperates with diminished DNA damage response to induce preleukemic stem cell expansion. Leukemia. 31(2):423-433.
The role of Fanconi Anemia (FA) proteins in hematopoiesis
Description
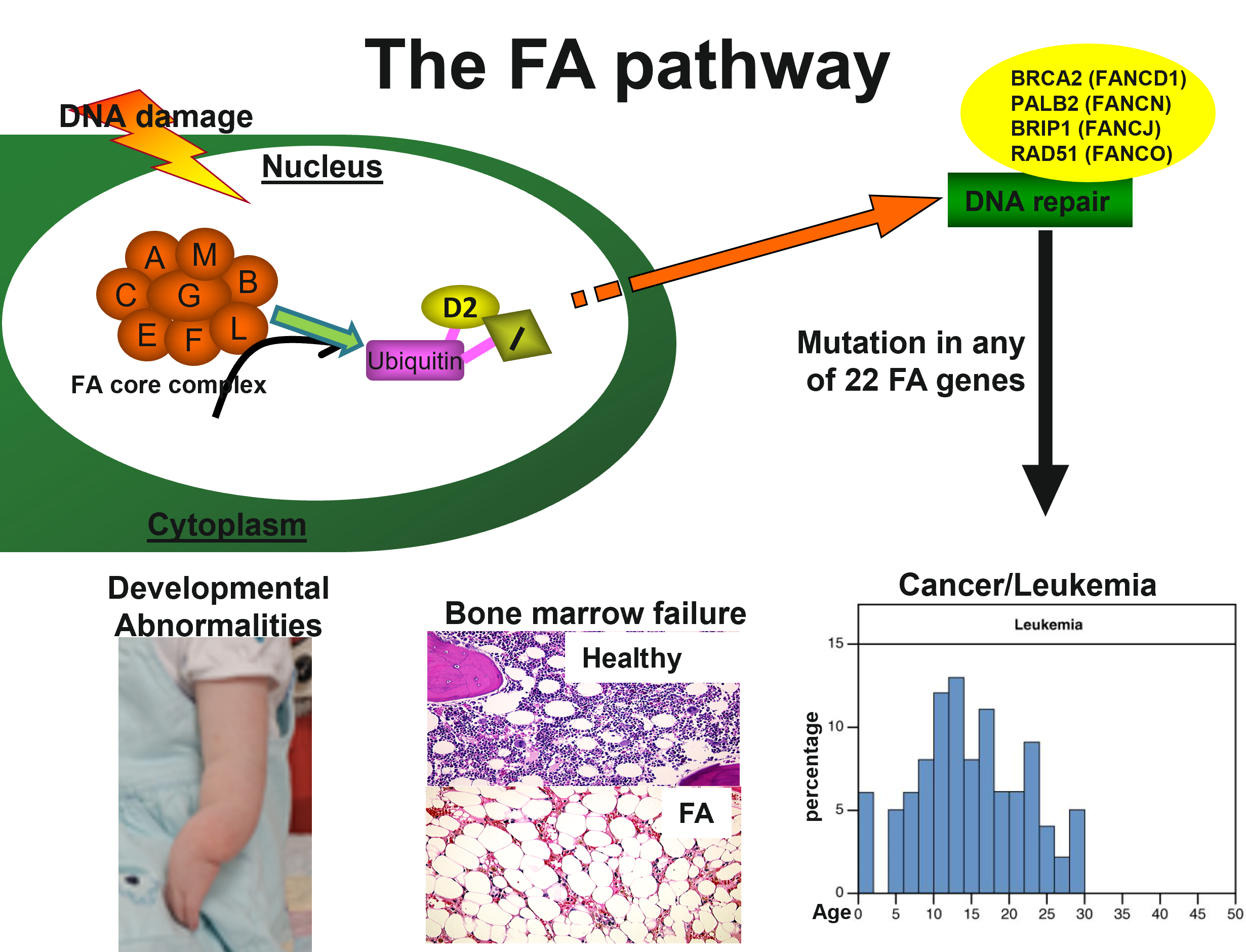
My laboratory focuses on elucidating the mechanisms by which the Fanconi anemia (FA) proteins regulate hematopoietic stem cells in the context of bone marrow failure (BMF) and leukemia development. The goal of my research is to identify novel pharmaceutical targets for the treatment of patients with FA and other bone marrow failure syndromes (BMFS).
The process of FA disease progression in the context of hematopoiesis is characterized by bone marrow failure, clonal proliferation of hematopoietic stem (HSC) and progenitor (P) cells, and progression to myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML). The only curable treatment for this devastating disease is stem cell therapy through bone marrow transplantation.Related publications
- Du W*, Erden O, Wilson A, Sipple J, Schick J, Mehta P, Myers KC, Davies SM, Steinbrecher K, Pang Q. (2014) Deletion of Fanca or Fancd2 dysregulates Treg in mice. Blood. 123(12):1938-1947.
- Du W*, Rani R, Sipple J, Schick J, Myer KC, Mehta P, Davies SM, Pang Q. (2012) The FA pathway counteracts oxidative stress through selective protection of antioxidant defense gene promoters. Blood. 119(18):4142-4151.
- Du W*, Li X, Sipple J, Pang Q. (2011) Overexpression of IL-3R on CD34+CD38- stem cells defines leukemia-initiating cells in FA AML. Blood. 117(16):4243-4252.
- Li J, Du W*, Pike S, Andreassen PR, Pang Q. (2009) Oxidative-stress specific interaction between FANCD2 and FOXO3a. Blood. 115(80):1545-1548.